Accelerating Pharmaceutical Factory Automation Using Digital Twin Technology
Pharmaceutical manufacturing is massive, difficult and unforgiving of careless processes which is exactly why it needs a better the global pharmaceutical market which about USD 1.64 trillion in 2024 and continues to grow so even the smallest inefficiency can turn into huge financial losses.
What makes pharmaceuticals different from traditional factory automation?

- Heavy regulatory requirements: In this industry, you cannot simply get your way through an audit
- Production that is organized batch by batch - every single batch carries its own legal, quality and financial implications
- Operations spread across many locations - production, testing, and clinical trials often happen in different laboratories and even across different countries.
This factors mean that a generic engineering tool will not work for pharmaceuticals. This approach must be centered on traceability, verified data and processes that keep track of events with time.
Consider Arun, a production manager at a midsized Indian pharmaceutical company. Is steam over 63 manufacturing facilities and often outsources specialized to drug batches to partners abroad. Last year, a single compliance gap in one outsourced batch cost the company millions and delayed FDA approval by 6 months. Arun’s problem was not as a result of lack of efforts but lack of visibility. He could not see what was happening across all sites in real time which made him not to have the capacity to expect issues before they happen.
This is where Digital Twin technologies becomes very important. It is not just a computer simulation but a live, data driven mirror of processes, equipment and even clinical trial groups.
In pharmaceuticals, strong digital twin systems help things monitor distance facilities, follow batches through every stage of production, and demonstrate compliance to auditors with boldness. This technology has already proven its ability to accelerate scale up, reduce the need for repeated experiment during development and improve operational decision making.
What is a Digital Twin in the Pharmaceutical Industry?
A digital twin in simple terms is a dynamic, data fed model that shows the behavior and state of a real system. It does not just sit idle like a stagnant char, rather it learns, update and moves at the exact system changes.
In pharmaceuticals, this means creating a virtual reflection of manufacturing plants, laboratory processes and even clinical trial groups. Unlike the standard simulation, a digital twin constantly receives new information from centers, production logs, quality reports and other systems. This allows you to behave like a “ live shadow” of operations.
Why Is This Powerful In Pharma?
Because every pill, vaccine or injectable carries a huge responsibility. A failure in one part of the system can destroy compliance, production cost and patient safety.
How It Works
- Reality Motivates All
All the information that comes from machines along with lab results and supply chains updates is integrated into the system. It acts like an augmented mirror of the company and shows what is happening right now.
- Everything is Visible.
No longer do dwindled managers have the option of report waiting. All they have to do is sign into the system and they have access to all processes across all facilities globally. There is no information that is hidden from them, and all reports are submitted on time, and all processes are completed on schedule.
- Avoid Issues Before They Arise.
The system focuses on risks and highlights those risks before they become an issue. Slow production lines, machines that do not work, and compliance issues have no bearing on the reporting that the system generates. Until issues arise across the board, the teams are notified, and no groundwork is left to be completed.
- No Risk Testing.
The equipment can be changed virtually before the actual equipment is touched. These types of changes are readily accessible, and do not waste physical material. Virtually all aspects of equipment work are optimized to avoid spending time, money, and efforts to minimize errors.
- Practical Demonstration.
Let’s assume there is a company wanting to increase the production of vaccines. It will be always late, and relies solely on lab reports that are collected on a manual basis, which are checked for a multitude of overlooked logs that are counter verified. Progress is always very slow, and errors are left unchecked.
With the twin system:
- Engineers are free to test new processes without first commencing the machines.
- Engineers no longer are forced to monitor machines and can greatly lower the work done on them.
- If a quality report is generated, and a batch is not, and something is set that is ‘off track’, the quality teams are then alerted.
- Compliance officers provide fully verified records to the regulators documenting exactly what happened.
This simply means that a pharmaceutical digital twin is not just about automation, it is about giving visibility, foresight and trust. It gives the manager control over complexity while ensuring the patient receives safe, reliable medicine.
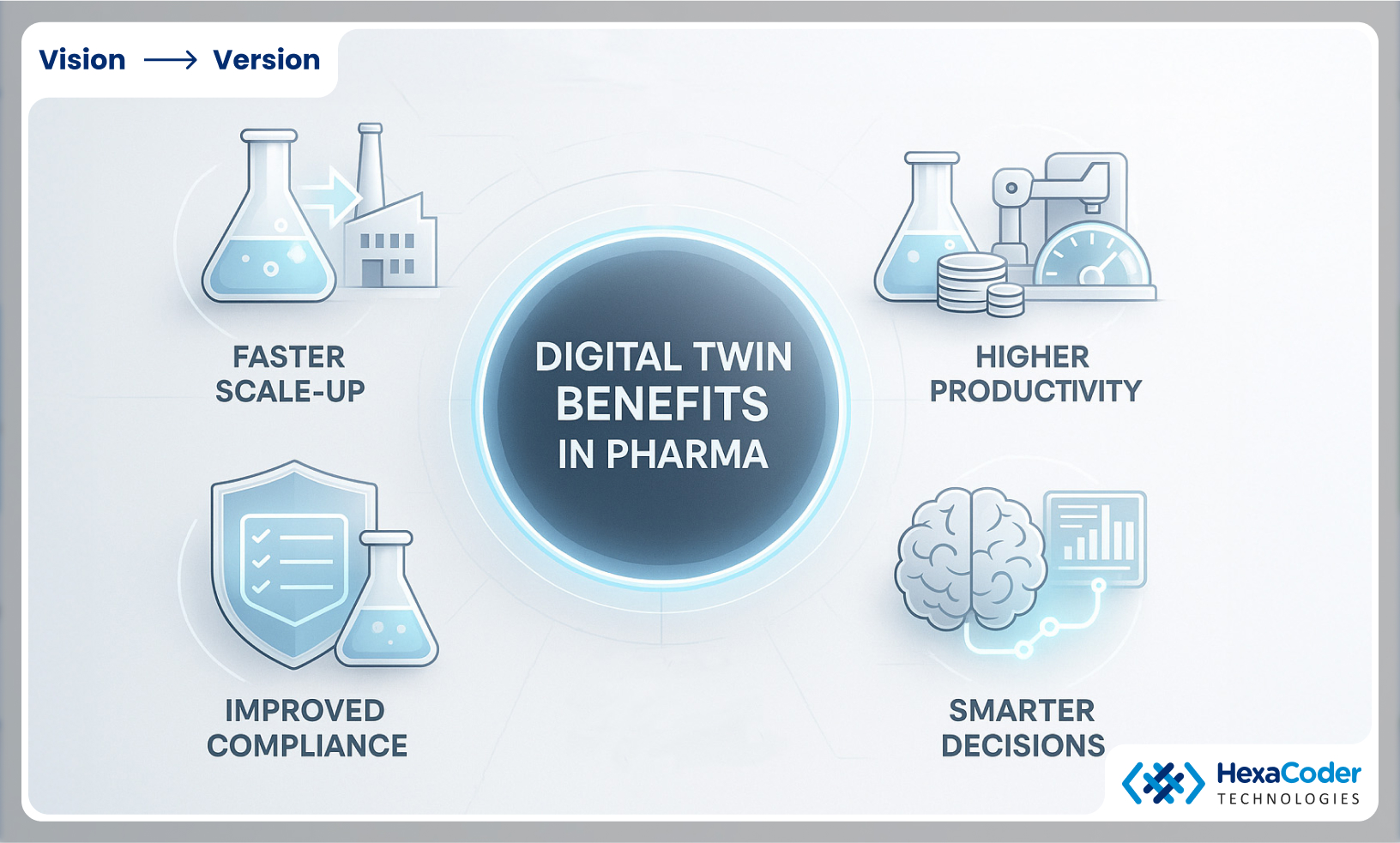
Important Benefits of Digital Twins in Pharma
Digital twins are not just fancy software, they also deliver real, measurable improvement to pharmaceutical operations for instance:
- Faster Scale Up
Moving from lab Discovery to full scale production takes years sometimes.
A digital twin allows tin to test processes changes virtually before applying them in the plant which saves month of trial and errors.
Example: A biotech firm scaling a new therapy can model temperature and mixing variables Italy which avoids dozens of expensive failed batches.
- Lower experiment costs:
Every lab experiment or batch trials consume valuable resources. With a digital twin, most of all these trials can be carried out in a virtual settings. Distance to reduce wasted raw materials, energy and time and allows scientists to focus on the most promising setups.
- Omproved Compliance Readiness
Regulators demand proof of every step from raw materials to the final packaging. A digital twin provides a complete and traceable record of batches, equipment use and quality checks. So instead of shaking before an audit, managers can confidently and over verified digital logs.
- Smart Decision Making:
Plant managers and R&D leads no longer need to wait on delayed reports. A digital to which shows insights that are up to date for example which machine is slowing down, which batch is trending of quality or which facility is falling behind. This feasibility drives a quick and better informed decision.
- Higher Uptime And Productivity
Equipment breakdowns in farmer can cause millions in lost revenue. So by predicting wear and performance problems, digital twins can schedule maintenance proactively. This can results to less downtime, more consistent output and few supply delays.
Digital twin applications across the three pharma verticals
Digital twins do not offer the same benefits for every part of pharma. Each vertical has unique challenges and parities and there is how the technology arts value where it matters the most.
Clinical Trials

Clinical trials at the backbone of pharmaceutical innovation, but they come with very difficult logistics, regulatory scrutiny, and high stakes. Digital twins can transform these processes by giving managers a real and actionable view of every trial batch and site.
Challenges
- Trailers involve multiple patients cohorts across locations which makes data tracking very difficult.
- Any delay, those errors or protocol deviation can cause an expensive setback.
- Compliance audit require every action and results to be traceable.
How Digital Twins Help
- Trial simulation before execution
Virtual patients can be modeled to predict outcomes, optimize dosage schedules and test trial protocols. For example, a cardiovascular drug trial can simulate five patient groups to see which dozing schedule produces the fastest and most effective results.
- Batch level tracking across sites
Every trial batch is monitored from preparation to administration. Multilocation trials can now be managed from a single dashboards so trial managers know which dashboard is at which site, what stage it is at and whether it meets protocol requirements.
- Simplified Regulatory Compliance
Digital twins automatically record all trial activities in an auditable format. Reports ethical regulatory agencies that generated immediately which reduces their amount of time spent on manual documentation.
- Predictive insights for trial and management:
By analyzing ongoing trial data, digital twins notes potential delays, patient dropouts or deviations in outcome before they happen which allows a proactive intervention
A Practical Example
Dr Meera, heading a multisight oncology trial in India and South East Asia makes use of digital twin to monitor five trials sites simultaneously. She can:
- See which batches are in transit, in preparation or administered.
- Identify if any site is lagging behind dosing schedules.
- Ensure compliance logs are up to date and ready for audit.
This results to trials being more efficient, compliance and predictable which actually reduces expenses, prevent delays while also improving patient safety.
Lab Testing Companies
Lab testing is a critical step in the pharmaceutical supply chain. Errors, delays, or missed results can affect product quality, regulatory compliance, and ultimately, patient safety. Digital twins give lab managers a complete view of operations across multiple labs, helping maintain precision and efficiency.
Challenges
- Managing large numbers of samples while ensuring strict accuracy.
- Meeting tight timelines for quality control and reporting.
- Regulatory audits require full traceability of every test, sample, and result.
- Oversight becomes difficult when labs operate in multiple locations.
How Digital Twins Help
- Virtual Experiment Testing
Lab procedures can be simulated to decrease errors, save resources, and establish most effective protocols before dealing with actual samples.
Illustration: A laboratory that is performing tests for a new antibiotic can simulate various scenarios, and sample preparation, and analyze which method is most likely to yield dependable outcome.
- Sample-Level Tracking Across Labs
Every sample is monitored from reception till the issuing of final results.
An interconnected system enables seamless connectivity across various facilities. Each location can access and analyze the status, location, and testing stage of each sample through a unified dashboard.
- Quality Assurance and Error Prevention
Results inconsistencies, risk of contamination, and equipment issues are detected and flagged early. An early warning system can be set in place to avoid repercussions of expensive errors that will be too late to rectify.
- Streamlined Compliance with Applicable Laws
All actions, observations, and results are captured in a logging system with approved formats.
No manual labor is needed to maintain records for an audit as all lost documentation is easily retrieved. This record keeping system is transparent and dependable.
Practical Example
Working with a network of laboratories that handles hundreds of samples on a daily basis across 3 different cities, a digital twin is utilized to monitor all operations at once. Lab managers can:
- Trace the testing phase and the position of each sample easily in real time.
- Recognize sites that are lagging timelines and are underperforming.
- Produce complete compliance logs for regulators without delay.
This means that laboratories operate efficiently, maintain high accuracy, and meet compliance standards consistently all while reducing wasted resources and preventing errors.
Production and Outsourced Manufacturing
Pharmaceutical production is where efficiency, quality, and compliance meet. Many companies operate multiple facilities, often in different countries, and some outsource specialized batches to external partners. Digital twins give managers a centralized, real-time view of all operations, making it possible to maintain quality and meet regulatory standards without physically visiting every plant.
Challenges
- Multiple production facilities, each with its own schedules, equipment, and staff.
- Every batch is legally, financially, and quality-wise unique.
- Outsourced manufacturing requires close oversight without direct control.
- Unexpected equipment problems or human errors can disrupt supply and lead to major financial loss.
How Digital Twins Help

- Unified Multi-Facility Dashboard
Managers can monitor all production sites alongside partners of record and see the status of every batch, equipment, and workflow stage in real time.
- Batch-Level Monitoring and Traceability
Every batch begins with raw materials and finished product, and every stage in the process can be reviewed. Any loss of quality, timing, and other production compliance are highlighted immediately.
Audit Inspections also receive a mastered batch from the Dissenting Mechanism, providing every detailed audio record comfortably established.
- Predictive Maintenance
Condition monitoring of a machinery aids in anticipating breakdown and takes charge of servicing schedules in a timed fashion to prevent down time and revenues loss.
- Remote Management and Decision Support
Quick intervention from the Executive Leadership can automatically be done and checkins can even be done from afar.
Process bottlenecks in teams make the improved decisions as well as resource compliance opportunities.
Practical Example
Arun is in charge of several in-house and outsourced production sites and utilizes a digital twin platform to:
- Forecast twenty production sequences in real time and monitor their performance metrics automatically, independently traversing across three main and two partner facilities.
- Recognize batch quality determiners two weak sequences prior and dozens of other step proxies in the workflow.
- Mentally formulate compliance review processes and automatically orchestrate in a ratio of several minutes to several days.
As the production starts to run smoothly across the targeted systems, all sites also move the batch quality progressively and managers have peace of mind knowing they can oversee operations remotely while avoiding costly disruptions.
Real Impact of Digital Twins Beyond Simulation.
Digital twins are often thought of as fancy simulations. In pharmaceuticals, they are so much more. They are living, operational tools that turn data into action, confusion into clarity, and complexity into control.
It matters because:
- Every decision in pharma affects patient safety, regulatory compliance, and revenue.
- Mistakes, delays, or inefficiencies quickly scale into major losses across multiple sites and batches.
- Digital twins give teams the power to see everything in one place, anticipate problems before they happen, and act with confidence.
Key Impacts Across Pharma
Total Visibility Across Operations
Whether it’s a clinical trial, lab network, or multiple manufacturing sites, managers can see every process, batch, and sample in real time.
Example: Arun can check five facilities and two outsourced partners at the same time, spotting bottlenecks or errors immediately.
Fast and Smart Decision Making
Teams no longer rely on delayed reports or guesswork. Insights from live operations allow instant course corrections, avoiding costly delays.
Example: A lab detects a deviation in sample preparation and corrects it before it affects results, saving thousands in wasted materials and hours of work.
Compliance and Audit Confidence
Every action, measurement, and result is automatically recorded and traceable. Auditors receive verified records instantly, reducing stress and manual paperwork.
Example: Regulatory inspections that used to take weeks can now be handled in hours with complete, verified batch histories.
Predictive Efficiency
Machines, workflows, and trials are monitored continuously, allowing problems to be anticipated and fixed before they happen.
This proactive approach reduces downtime, improves batch quality, and accelerates time to market.
Scale Without Chaos
Whether a company is expanding trials, opening new labs, or outsourcing production, digital twins allow consistent quality and oversight at every stage.
No matter how complex the network, teams can manage operations as if everything were in a single, controlled environment.
A Vivid Example
Consider a pharma company rolling out a new vaccine:
- Five plants in different cities produce multiple batches, while labs across regions handle testing and quality assurance.
- Using a digital twin, managers monitor batch progress, lab results, and equipment health from one central dashboard.
- Predictive insights flag a production line running slightly off-spec. The team intervenes immediately, preventing a costly batch failure.
- Auditors review complete, traceable records for every batch without disruption.
The Outcome is: Faster production, higher compliance confidence, fewer errors, and better visibility at every level. Teams work smarter, patients benefit, and the business grows without friction.
In this line of work, the use of digital twins is a strategic necessity and not an option. Their ability to simplify the most convoluted and multifaceted situation is unparalleled. Equally, they have the capacity to mitigate the most abstract forms of information and transform them into a decisive action. Accomplishing all of those translates to lower costs and faster results. Regulatory confidence is higher and patient safety is significantly put at the forefront.
Take Control, Stay Ahead.
Pharmaceuticals is an industry that lacks the toleration of mistakes. Every lab decision, every batch, and every trial is critical. Digital twins allow you to transform uncertainty and ambiguity into clarity and control.
- Monitor and control all facilities, processes, and outcomes in real-time.
- Predict delays, avoid operational bottlenecks, and smoothen all flowing processes.
- Always be takeover ready. Compliant, management of active processes is systematic supervision.
- Stay in control by expanding all processes. Trials, labs, or mass production facilities.
It is obvious that companies that have incorporated digital twins as part of their strategy are the only ones that are thriving. They enjoy faster time to market, a higher reduction in waste and costs, and have the most rigorous standards of quality and compliance at every level.
Your next step: Plan to shatter every barrier of growth and opportunity. See every batch, every process, and every facility all in one frame. Work with Hexacoder Technologies and see just how digital twins can transform your business.





